Quality Improvement
Research or Quality Improvement? Breaking Down the Differences
Kathleen Bickel, MD MPHIL MS, and Amy L. Davis, DO MS FACP FAAHPM
Improving palliative care practice—is my project idea quality improvement (QI) or is it research?
The practice of hospice and palliative care always has been about improving quality one patient-family unit at a time. However, a project undertaken to “improve quality” is not always a QI project—it might be research. QI involves “systematic, data-guided activities designed to bring about immediate improvements in healthcare delivery in particular settings.”1 Meanwhile, research involves “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.”2 Although helpful, these definitions are not absolute. For example, pilot research may not be generalizable, and QI work might be generalizable to similar programs elsewhere.
Consider two common examples, a randomized controlled clinical trial (RCT) and a local QI project (Table 1). An RCT focuses on testing the intervention and generating new scientific knowledge. The gap in research is between what we know and what we do not yet know. The goal is to test the hypothesis and test the new intervention against the current standard of care or a placebo. The research protocol is prespecified, requires Institutional Review Board review and patient consent, and generally is not altered after study initiation. Randomization and blinding are used to decrease confounding and bias. Interim safety analyses occur, but most data analysis occurs only after study completion to avoid causing data interpretation bias.
In contrast, QI focuses on providing high-value health care that is safe, effective, person-centered, timely, equitable, and efficient.3 QI projects are designed specifically to improve and sustain local clinical system performance while implementing changes within current care standards. The standards for care (eg, clinical guidelines) are known, but may not be well implemented. The gap in QI is between what we know and what we do. The goal for a QI project is often reflected in a SMART aim: Specific, Measurable, Attainable, Reasonable, and within a specified Timeframe. To meet the SMART aim, the process targeted for change is analyzed and “change ideas” are solicited from local stakeholders and the QI project team. Change typically is implemented using a specific change process, such as PDSA (Plan-Do-Study-Act) cycles, Lean, Six Sigma, DMAIC (Define, Measure, Analyze, Improve and Control), etc.
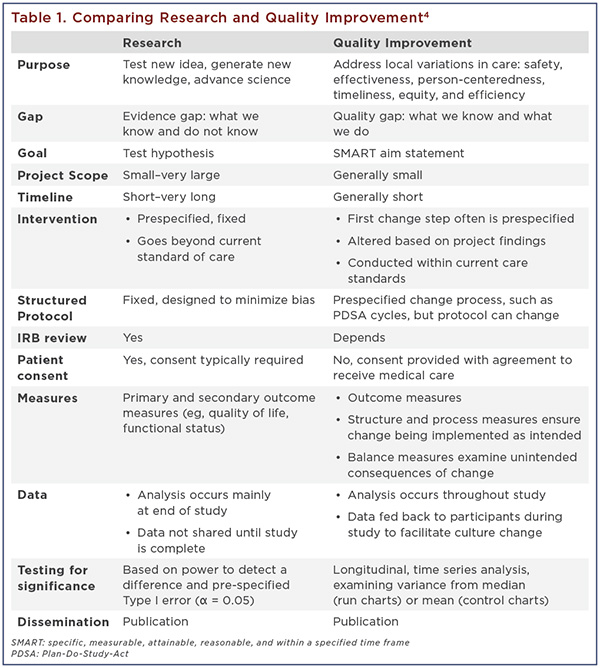
Unlike an RCT, QI is an incremental process involving multiple tests of change over time. Smaller amounts of data are examined more frequently and over time using statistical probability to detect nonrandom variation over time or a “special cause” signal. Learning from the data occurs in real time, enabling us to change the protocol or intervention as needed based on insights from the data and local experiences.
Although other types of research and QI projects exist, this brief overview highlights some of the major differences. For more information and resources on QI in hospice and palliative care, please see aahpm.org/quality. We encourage you to consider submitting your work as an abstract during the 2020 Annual Assembly Call for Scientific and Quality Improvement proposals, which will be open from July 1–July 31, 2019.
Kathleen Bickel, MD MPhil MS, is a National Institute of Aging T32 Post-Doctoral Research Fellow at the University of Colorado School of Medicine and practices as an outpatient palliative care physician at the Rocky Mountain Veterans Affairs Medical Center. She has been serving as a Quality Improvement representative to the Annual Assembly Scientific and Quality Improvement Subcommittee since 2018 and is also a member of the AAHPM CMS MACRA TECUPP and MSP Panels. She can be reached at This email address is being protected from spambots. You need JavaScript enabled to view it. .
Amy L. Davis, DO MS FACP FAAHPM, is an independent palliative care physician in the Philadelphia region. She is also board certified in addiction medicine and a Clinical Associate Professor at Drexel University. Davis co-chairs the AAHPM Quality Committee and the AAHPM/HPNA QI Education Working Group and serves on the CMS MACRA TECUPP. She can be reached at This email address is being protected from spambots. You need JavaScript enabled to view it. .
References
- Lynn J, Baily MA, Bottrell M, et al. The ethics of using quality improvement methods in health care. Ann Intern Med. 2007;146(9):666–673.
- Title 45, public welfare, part 46, protection of human subjects. Fed Regist. 2011;128–148. Codified at §46.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- Kamal, A. Improving the quality of care: a practical skill-building workshop. Proceedings from the Annual Assembly of Hospice & Palliative Care; March 13–16, 2019; Orlando, FL.
Read the next article or go to the table of contents.
